Showcase Your Innovations & Technologies
This informal, interactive session is designed to help you connect with fellow innovators, scientists, regulatory specialists, and R&D leaders shaping the future of early‑life nutrition. With an audience deeply focused on ingredient science, safety substantiation, regulatory readiness, and functional performance in formula, you’ll have the opportunity to present a poster highlighting your own research, data, or technological breakthroughs.
Don’t miss the chance to deepen relationships, exchange insights, and position your work at the centre of the industry’s most forward‑looking discussions.
- Demonstrate your expertise in advancing novel ingredients, safety evidence, efficacy data, or manufacturing innovation for infant formula
- Gain targeted visibility among formula manufacturers, ingredient developers, regulatory experts, and early‑life nutrition scientists
- Engage in meaningful, technical discussions and gather direct feedback to strengthen your scientific or regulatory narrative
- Attract interest from potential partners, collaborators, and commercial stakeholders seeking promising new ingredients or technologies
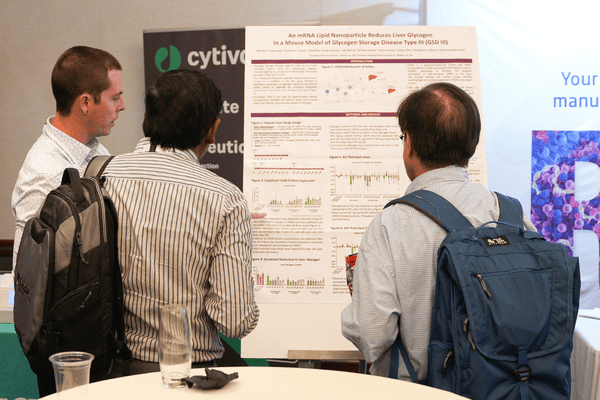


How to Submit Your Poster
How to submit your poster
- You must first register your place to attend the Novel Ingredients for Infant Formula Summit prior to submitting a poster
- Submit a short abstract for review to be considered for inclusion in the Scientific Poster Session
- Once accepted, you will receive confirmation along with printing guidelines, dimensions, and on‑site setup details. A PDF version of your poster will also be required 2 weeks before the summit
- Scientific Poster Session: June 10th, 2026, 5.05pm
- Abstract Submission Deadline: X